A) Disulfide linkages
B) Hydrogen bonds
C) Salt bridges
D) Hydrophobic interactions
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nearly all amino acids immediately release N2 gas upon diazotization (treatment with aqueous HCl and sodium nitrite) .Which amino acid would not?
A) Lysine
B) Histidine
C) Tyrosine
D) Cysteine
E) Proline
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which amino acid is responsible for the condition known as phenylketonuria (PKU) which occurs in a small fraction of the population?
A) Tryptophan
B) Histidine
C) Phenylalanine
D) Proline
E) Valine
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which amino acid does not form a characteristic blue color with ninhydrin?
A) Cysteine
B) Lysine
C) Proline
D) Valine
E) Tryptophan
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the additional (ring) nitrogen in tryptophan not very basic? 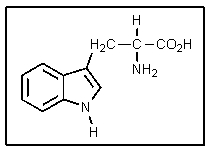
A) Cyclic amines are not basic.
B) It is too far from the carboxyl group to be effected.
C) Aromatic amines are weakly basic because of resonance.
D) The additional amine group is offset by an additional carboxylic acid group.
E) It is sp2 hybridized as therefore not as basic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The secondary structure of proteins is held together by hydrogen bonds between which protein sub-structures?
A) The C=O and N-H on amino acid side chains
B) The C=O on the protein backbone and the N-H on the amino acid side chains
C) The C=O on the amino acid side chains and the N-H on the protein backbone
D) The C=O and the N-H on the protein backbone
E) There are no hydrogen bonds involved with secondary structure.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What process would one use to make multiple copies of a fragment of DNA?
A) Polymerase chain reaction
B) Electrophoresis
C) Transcription
D) Translation
E) Merrifield solid-phase synthesis
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Folding of polypeptides influenced by distant residues is an example of what protein structure?
A) Primary
B) Secondary
C) Tertiary
D) Quaternary
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the abbreviated name of the following polypeptide? 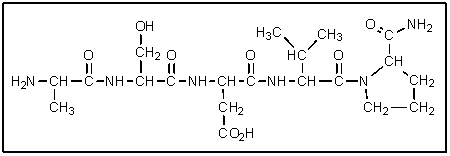
A) val-thr-glu-ala-gln
B) ala-thr-glu-val-gln
C) met-ser-asp-leu-pro-NH2
D) ala-ser-asp-val-gln
E) ala-ser-asp-val-pro-NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would result from the following reactions? 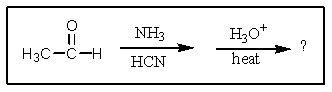
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is least likely to be found in nature?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents how a typical amino acid would mainly exist in basic solution (pH > 7) ?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is the following not a good two step route for the preparation of pure L-alanine? 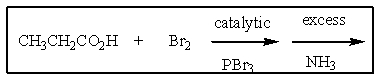
A) The bromination is not selective (the CH3 and CH2 groups are both brominated) .
B) Ammonia is not sufficiently nucleophillic to react with a secondary bromide.
C) Only the CH3 group is brominated.
D) The product will be racemic.
E) Propanamide would be the major product.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which amino acid does not have an enantiomer?
A) Alanine
B) Glutamine
C) Histidine
D) Aspartic acid
E) Glycine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Chymotrypsin catalyzes hydrolysis of peptide bonds where? 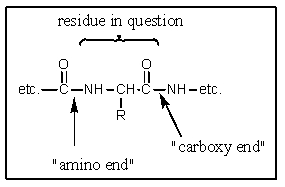
A) At the carboxy end of residues with basic side chains
B) At the amino end of residues containing aliphatic residues
C) At the carboxy end of residues with aromatic side chains
D) At the amino end of residues with aromatic side chains
E) At the carboxy end of methionine residues
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the DNA base shown? 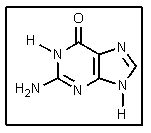
A) Guanine
B) Cytosine
C) Adenine
D) Uracil
E) Thymine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It was not until 1974 that -carboxyglutamic acid (shown below) was discovered (by researchers at the University of Colorado) in polypeptides.Based on your knowledge of organic chemistry,what is the most likely reason that this particular amino acid escaped identification so long? 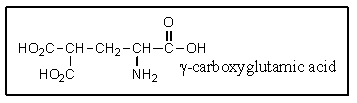
A) It tended to spontaneously form a cyclic anhydride:![]()
B) It tended to spontaneously lose carbon dioxide:![]()
C) It easily underwent a reverse-Knovenagle reaction:![]()
D) It tended to spontaneously form a cyclic amide (lactam) :![]()
E) It was simply too polar to extract into non-polar organic solvents.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is dicyclohexylcarbodiimide (DCC) used for in peptide synthesis?
A) DCC protects the amino group of the intended N-terminal amino acid.
B) DCC activates the carboxyl group toward nucleophillic attack.
C) DCC cleaves the blocking groups from the final peptide.
D) DCC is the resin (solid support) used to anchor the growing polypeptide.
E) DCC removes the peptide from the resin at the conclusion of the synthesis.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Amino acids are covalently bonded to each other through_______.
A) hydrogen bonding
B) disulfide bridges
C) peptide bonds
D) ( and folds)
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Although traces of racemic amino acids have been found in meteorites,nearly all naturally occurring amino acids on Earth have the stereochemistry shown.What is true of this stereochemistry? (Assume R = alkyl) 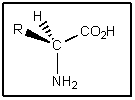
A) Amino acids are of the (R) configuration.
B) Amino acids are of the (S) configuration.
C) Amino acids are not chiral.
D) Amino acids are meso compounds.
E) Amino acids easily change configuration.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 39
Related Exams