A) square pyramidal
B) square planar
C) see-saw
D) octahedral
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to decide which one of the following molecules and ions will have a trigonal pyramidal geometry. (The central atom is always first in the formula.)
A) PCl3
B) BF3
C) SO3
D) BrF3
E) CO32-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX5 will have a ______ molecular shape.
A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX3 will have a ______ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following Lewis structures is definitely incorrect?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of SCl3F as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in GeCl4 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) < 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a net dipole moment?
A) BeCl2
B) SF2
C) KrF2
D) CO2
E) CCl4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of ClF2- as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) L-shaped
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX2E will have a ______ molecular shape.
A) bent
B) see-saw
C) trigonal planar
D) T-shaped
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of the thiocyanate anion, SCN-, as predicted by the VSEPR theory? (Carbon is the central atom.)
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX3E will have a _____ molecular shape.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) triangular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in FNO using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) between 120 and 180°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 that minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.)
A) BrF3
B) H3O+
C) PCl3
D) SO42-
E) SF4
Correct Answer

verified
Correct Answer
verified
True/False
All possible resonance structures contribute equally to the resonance hybrid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following does the nitrogen atom have a formal charge of -1?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in AsCl3 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) between 110 and 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following species is the central atom (the first atom in the formula) likely to violate the octet rule?
A) BF4-
B) XeO3
C) SiCl4
D) NH3
E) CH2Cl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of N2O as predicted by the VSEPR theory? 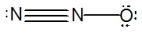
A) trigonal pyramidal
B) trigonal planar
C) angular
D) bent
E) linear
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 98
Related Exams