A) H₂S
B) CO₂
C) CH₄
D) H-C ![]() C-H
C-H
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dipole-induced dipole forces of attraction exist between water and gasoline, and yet these two substances do not mix because water has such a strong attraction for itself. Which of the following compounds might best help to make these two substances mix into a single liquid phase? 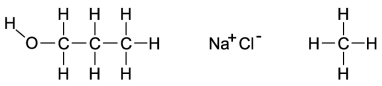
A) the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B) the molecule in the middle because when the salts mix into the water, it will help separate the water and decrease the attraction for itself
C) The molecule on the right will form attractions with the polar ends of the water, allowing the gasoline a chance to mix with the water.
D) All of these molecules would be equally effective at increasing the mixing of gasoline and water.
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements will most likely form an ion with a -2 charge?
A) Na
B) S
C) Ne
D) Mg
E) Cl
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes an aqueous solution?
A) a mixture of some compound dissolved in water
B) a mixture of polar molecules dissolved in a nonpolar solvent
C) a mixture of water dispersed in an ionic compound
D) a mixture of nonpolar molecules dissolved in a polar solvent
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is most likely to show a dipole-dipole interaction?
A) CH₃OH
B) CH₃SH
C) CH₄
D) H-C ![]() C-H
C-H
E) A and B
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? swimming pool water
A) homogeneous mixture
B) heterogeneous mixture
C) a pure element
D) a pure compound
E) depends on how many children have been in it
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the concentration of gold in seawater is 2.0 milligram per ton of sea water and the mass of the ocean is 1.5 × 1018 tons, how much gold is in the ocean?
A) 3) 0 × 1012 kg
B) 3) 0 kg
C) 300 g
D) 36 mg
E) 3,000 lb
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of sucrose are in a 0.5 moles of sucrose?
A) 3) 01 × 1023 molecules of sucrose
B) 6) 02 × 1023 molecules of sucrose
C) 12.04 × 1023 molecules of sucrose
D) 0) 5
E) 1 gram
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The valence electron of a sodium atom does not sense the full +11 of the sodium nucleus. Why not?
A) There are two "non-valence shell" electrons shielding the sodium nucleus from sensing it.
B) There are two inner shells of electrons containing ten electrons shielding the sodium nucleus from sensing it.
C) Since the +11 charge is spread evenly around the entire spherical surface of the nucleus, the actual force of the charge in any given direction is greatly diminished.
D) The distance from the nucleus to the loosely held lone valence electron varies greatly over time. So, the average sense of charge from the nucleus is considerably less than +11.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the main form of intermolecular attractions among water molecules?
A) hydrogen bonding
B) induced dipole-induced dipole
C) covalent bonding
D) ion-dipole
E) polar-induced polar
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? a cappuccino (with foam)
A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What can be said about drinking water that is 99.9999 percent free of some poison, such as a pesticide?
A) In each 10,000 parts of the contaminated water there is one part pesticide and 9999 parts pure water.
B) In each 100,000 parts of the contaminated water there is one part pesticide and 99,999 parts pure water.
C) The ratio of water molecules to pesticide molecules in the glass is so great that drinking the water is not problematic.
D) The water is highly contaminated and surely not fit to drink.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is the most dilute?
A) 0) 1 liter of water with 1 gram of sugar
B) 0) 2 liter of water with 2 grams of sugar
C) 0) 5 liter of water with 5 grams of sugar
D) 1 liter of water with 10 grams of sugar
E) They all have the same concentration.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does oxygen have such a low solubility in water?
A) Water's attraction for itself is stronger than its attraction for oxygen molecules.
B) Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C) The hydrogen bonding in water keeps the oxygen solubility low.
D) Both A and B are true.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the sum of the atomic masses of all the atoms in sucrose, C₁₂H₂2O11?
A) 342 amu
B) 182 amu
C) 270 amu
D) none of the above
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? coffee (black)
A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nonbonding pairs of electrons are in the following molecule? H-H
A) 1 pair
B) 6 pairs
C) 0 pairs
D) 8 pairs
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on atomic size, which would you expect to be more soluble in water: helium, He, or nitrogen, N2?
A) Although He is smaller, its outer orbital is filled and the atom will have little attraction to the water molecules.
B) Since He atoms are smaller, more of them can fit into solution, so it has a higher solubility in water.
C) Nitrogen atoms are bigger and so nitrogen molecules should be more soluble in water due to greater dipole-induced dipole attractions.
D) He atoms are bigger and so helium molecules should be more soluble in water due to greater dipole-induced dipole attractions.
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why does oxygen have such a low solubility in water?
A) Water's attraction for itself is stronger than its attraction for oxygen molecules.
B) Water and oxygen only attract one another by means of weak dipole-induced dipole attractions.
C) The hydrogen bonding in water keeps the oxygen solubility low.
D) Both A and B are true.
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is untrue?
A) Covalent molecules are never crystalline.
B) Covalent molecules usually have low melting points.
C) Covalent molecules can have nonbonding electrons.
D) Covalent bonds can involve more than one pair of electrons.
E) All of the above statements are true.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 248
Related Exams