A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar solubility of SrF2 in water is 8.8×10 - 4 M. What is the solubility product constant, K sp, for this substance?
A) K sp = 6.81×10 - 10
B) K sp = 2.73×10 - 9
C) K sp = 7.74×10 - 7
D) K sp = 1.55×10 - 6
E) K sp = 2.64×10 - 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25 C, K p = 0.240 for the reaction:
2 ICl (g) 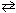 I2 (g) + Cl₂ (g)
If 2.66 atm of ICl are placed in a 1.0 L flask, what is the equilibrium pressure of I2 ?
I2 (g) + Cl₂ (g)
If 2.66 atm of ICl are placed in a 1.0 L flask, what is the equilibrium pressure of I2 ?
A) 0.658 atm
B) 1.52 atm
C) 8.33 atm
D) 0.585 atm
E) 0.24 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of Ag2SO4 in the presence of 0.22 M aqueous solution of AgNO3? K sp(Ag2SO4) = 1.5×10 - 5
A) [Ag2SO4] = 7.8×10 - 5 M
B) [Ag2SO4] = 1.6×10 - 4 M
C) [Ag2SO4] = 3.1×10 - 4 M
D) [Ag2SO4] = 6.1×10 - 4 M
E) [Ag2SO4] = 1.6×10 - 2 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the reversible reaction, N₂+ O₂ 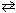 2 NO
Has reached a state of dynamic equilibrium , which statement(s) below is(are) true?
I. Both the forward and reverse reactions shut down and no more NO, N₂or O₂are produced.
II. The rate of the forward reaction equals the rate of the reverse reaction.
III. The rate constant of the forward reaction equals the rate constant of the reverse reaction.
2 NO
Has reached a state of dynamic equilibrium , which statement(s) below is(are) true?
I. Both the forward and reverse reactions shut down and no more NO, N₂or O₂are produced.
II. The rate of the forward reaction equals the rate of the reverse reaction.
III. The rate constant of the forward reaction equals the rate constant of the reverse reaction.
A) I only
B) II only
C) III only
D) I and II
E) All of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the effect upon addition of Argon (an inert gas) to the equilibrium reaction shown below?
N₂O4 (g) 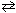 2 NO₂ (g)
2 NO₂ (g)
A) No effect
B) The reaction shifts to the right.
C) The reaction shifts to the left.
D) More information is needed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions is product favored by a higher temperature ?
I. N₂(g) + 2 O₂+ Heat 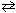 2 NO₂ (g)
II. 2 N₂(g) + 6 H₂ O (g)
2 NO₂ (g)
II. 2 N₂(g) + 6 H₂ O (g) 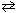 4 NH₃(g) + 3 O₂(g) (endothermic reaction)
III. C₂ H4 (g) + 3 O₂(g)
4 NH₃(g) + 3 O₂(g) (endothermic reaction)
III. C₂ H4 (g) + 3 O₂(g) 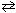 2 CO₂ (g) + 2 H₂ O (g) ( D H 0)
2 CO₂ (g) + 2 H₂ O (g) ( D H 0)
A) I only
B) II only
C) III only
D) I and II
E) I and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the partial pressure equilibrium constant, K p , for the following reaction if the concentration equilibrium constant K c = 7.17*1015 at 200 K?
N₂(g) + 3 H₂ (g) 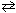 2 NH₃(g)
2 NH₃(g)
A) K p = 7.21×10 - 35
B) K p = 2.66×1013
C) K p = 4.37×1014
D) K p = 1.18×1017
E) K p = 1.93×1018
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium governed by the reaction 4 NH₃(g) + 5 O₂(g) 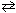 4 NO (g) + 6 H₂ O (g) undergoes a change in volume from 1.0 L to 2.0 L at 600 C. Choose the correct statement below.
4 NO (g) + 6 H₂ O (g) undergoes a change in volume from 1.0 L to 2.0 L at 600 C. Choose the correct statement below.
A) The number of moles of H2O decreases.
B) The number of moles of NO remains the same.
C) The number of moles of NH3 decreases.
D) The value of K c increases.
E) The number of moles of both H2O and O2 decrease.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A simplifying assumption can be made when solving problems in equilibrium which assumes that "x" is considerably small (approaches zero) in the sum or difference of an expression. As such, it drops out of the expression and the Algebra becomes much easier. When is it valid to use a simplifying assumption?
A) It is valid to make this assumption when the ratio: "x"/[reactant A]o * 100% > 5%
B) It is valid to make this assumption when 95 out of 100 scientists chose to do so.
C) It is valid to make this assumption when "x" is comparable in magnitude to the initial concentration of the reactant A. (i.e., x ~ [A]o)
D) It is valid to make this assumption when the ratio: "x"/[reactant A]o < 5
E) It is valid to make this assumption when the ratio: "x"/[reactant A]o * 100% < 5%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ionic compound(s) listed below would increase in solubility upon lowering the pH? I. CaF2 II. Ni(OH) 2 III. PbCl2
A) I only
B) II only
C) III only
D) I and II
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of SO₂ , two moles of O₂, and two moles of SO3 are placed in a 1.0 liter flask. If K c for the reaction 2 SO₂ (g) + O₂(g) ![One mole of SO₂ , two moles of O₂, and two moles of SO<sub>3</sub> are placed in a 1.0 liter flask. If K c for the reaction 2 SO₂ (g) + O₂(g) 2 SO<sub>3</sub>(g) is 0.50 at this temperature, what occurs in the flask as equilibrium is approached? A) [SO<sub>3</sub>] increases B) [O<sub>2</sub>] increases C) [SO<sub>2</sub>] decreases D) No reaction occurs since the system is at equilibrium. E) The equilibrium concentrations of [O<sub>2</sub>] and [SO<sub>2</sub>] are very small.](https://d2lvgg3v3hfg70.cloudfront.net/TBX8714/11ebff50_c8b0_84ec_8da6_8fc35af5f8a0_TBX8714_11.jpg) 2 SO3(g) is 0.50 at this temperature, what occurs in the flask as equilibrium is approached?
2 SO3(g) is 0.50 at this temperature, what occurs in the flask as equilibrium is approached?
A) [SO3] increases
B) [O2] increases
C) [SO2] decreases
D) No reaction occurs since the system is at equilibrium.
E) The equilibrium concentrations of [O2] and [SO2] are very small.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution is prepared such that the concentrations of the ions F - , Cl - , Br - , and I - are all 0.0010 M . Solid Pb(NO3) 2 is added to the solution. Which insoluble lead salt precipitates last? PbF2 K sp = 3.7×10 - 8 PbCl2 K sp = 1.7×10 - 5 PbBr2 K sp = 6.3×10 - 6 PbI2 K sp = 8.7×10 - 9
A) PbI2
B) PbBr2
C) PbCl2
D) PbF2
E) impossible to determine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In water saturated with BaF2 at a particular temperature, the equilibrium concentration of fluoride ions is 1.5×10 - 2 M . What is the K sp of barium fluoride?
A) 1.7×10 - 6
B) 2.2×10 - 4
C) 9.0×10 - 4
D) 3.4×10 - 6
E) 4.6×10 - 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2 NaHSO₄(s) 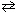 Na2 S2O 7 (s) + H₂ O (g) is endothermic at 298 K. Therefore,
Na2 S2O 7 (s) + H₂ O (g) is endothermic at 298 K. Therefore,
A) D H ° rxn is positive.
B) The partial pressure of H2O would be higher at 500K.
C) K p would increase with increasing temperature.
D) All of a, b, and c are correct.
E) None of a, b, and c are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
K c = 0.35 for the reaction:
H₂ (g) + I2 (g) 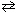 2 HI (g)
If one mole of H₂ and one mole of I2 are placed in a 1.0 liter container, the equilibrium concentration of HI is:
2 HI (g)
If one mole of H₂ and one mole of I2 are placed in a 1.0 liter container, the equilibrium concentration of HI is:
A) 0.46 M
B) 0.77 M
C) 0.23 M
D) 0.63 M
E) 0.30 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant for the reaction below is 1.7*1017 at 25 C.
H₂ (g) + Br₂(g) 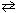 2 HBr (g) K c (25 C) = 1.7*1017
What can be stated about the relative equilibrium distribution for this mixture?
2 HBr (g) K c (25 C) = 1.7*1017
What can be stated about the relative equilibrium distribution for this mixture?
A) The mixture contains predominantly H2 at equilibrium.
B) The mixture contains predominantly Br2 at equilibrium.
C) The mixture contains predominantly HBr at equilibrium.
D) The mixture contains predominantly H2 and Br2 at equilibrium.
E) The mixture contains relatively equal amounts of H2, Br2 and HBr at equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a certain temperature K c = 9.0 for the equilibrium N₂O4 (g) 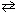 2 NO₂ . What is K c at the same temperature for:
NO₂ (g)
2 NO₂ . What is K c at the same temperature for:
NO₂ (g) 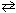 1 / 2 N₂O4 (g)
1 / 2 N₂O4 (g)
A) 1.1
B) 3.0
C) 0.33
D) 9.0
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following system at equilibrium. A reaction vessel is charged witH₂ .46 atm of NO, 2.46 atm of H₂ O and 1.23 atm of H₂ gases. Equilibrium is established as shown in the reaction below. 2 NO (g) + 2 H₂ (g) 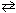 N₂(g) + 2 H₂ O (g) At equilibrium, the partial pressure of NO is 1.53 atm. What is the equilibrium partial pressure of N₂gas in this mixture?
N₂(g) + 2 H₂ O (g) At equilibrium, the partial pressure of NO is 1.53 atm. What is the equilibrium partial pressure of N₂gas in this mixture?
A) 0.00 atm
B) 0.465 atm
C) 0.765 atm
D) 0.930 atm
E) 1.86 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The K sp for PbBr2 is 4.0×10 - 5 at a particular temperature. What is the solubility of PbBr2 in pure water?
A) 6.3×10 - 3 M
B) 2.2×10 - 2 M
C) 3.4×10 - 2 M
D) 4.0×10 - 5 M
E) 3.2×10 - 3 M
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 212
Related Exams