A) 0. 00543 mol
B) 0. 0870 mol
C) 11.5 mol
D) 184 mol
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound with covalent bonds.
A) CH4
B) Kr
C) KBr
D) K
E) NaCl
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of chlorine gas?
A) 35.5 g/mol
B) 70.9 g/mol
C) 6.02 × 1023 g/mol
D) 1.20 × 1023 g/mol
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) An ionic bond is much stronger than most covalent bonds.
B) An ionic bond is formed through the sharing of electrons.
C) Ionic compounds at room temperature typically conduct electricity.
D) Once dissolved in water, ionic compounds rarely conduct electricity.
E) None of the above are true.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical formula for copper( II) phosphate?
A) Cu2P
B) Cu2PO4
C) Cu3P2
D) Cu3( PO4) 2
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the chemical formula for magnesium hydroxide?
A) MgH2
B) MgOH
C) MgOH2
D) Mg(OH) 2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of carbon are contained in 47.6 g of Al2(CO3) 3? The molar mass of Al2(CO3) 3 is 233.99 g/mol.
A) 1.23 × 1023 C atoms
B) 2.96 × 1024 C atoms
C) 2.87 × 1025 C atoms
D) 1.10 × 1024 C atoms
E) 3.68 × 1023 C atoms
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following exists as a diatomic molecule?
A) hydrogen
B) carbon
C) phosphorus
D) calcium
E) xenon
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following contains 35% carbon by mass?
A) C2H2
B) CH4
C) CH3F
D) CO2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula for zinc chloride?
A) ZnC
B) Zn2Cl2
C) ZnCl2
D) Zn2Cl
E) ZnCl3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is ethanol?
A) C2H6
B) C2H5OH
C) CH3CO2H
D) CH3CO2CH3
E) CH3OCH3
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the name for F2O.
A) fluorine oxide
B) di fluorine monoxide
C) fluorine (I) oxide
D) fluorine (II) oxide
E) fluorate
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the name for H2CO3.
A) carbonous acid
B) dihydrogen carbonate
C) carbonic acid
D) hydrocarbonic acid
E) hydrocarbide acid
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions is associated with the lattice energy of Li2O (ΔH°latt) ?
A) Li2O(s) → 2 Li⁺(g) + O2⁻(g)
B) 2 Li⁺(aq) + O2⁻(aq) → Li2O(s)
C) 2 Li⁺(g) + O2⁻(g) → Li2O(s)
D) Li2O(s) → 2 Li⁺(aq) + O2⁻(aq)
E) 2 Li(s) + ![]() O2(g) → Li2O(s)
O2(g) → Li2O(s)
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A single covalent bond contains ________ of electrons.
A) 0 pairs
B) 1 pair
C) 2 pairs
D) 3 pairs
E) 4 pairs
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass for Mg(ClO4) 2.
A) 223.21 g/mol
B) 123.76 g/mol
C) 119.52 g/mol
D) 247.52 g/mol
E) 75.76 g/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solid compound,Na4SiO4,contains
A) Na+, Si4+, and O2- ions.
B) Na+ and SiO4 -4 ions.
C) Na4+ and SiO4 -4 ions.
D) Na4SiO4 molecules.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the name for NaHSO3.
A) sodium sulfite
B) sodium sulfate
C) sodium hydrogen sulfate
D) sodium hydrogen sulfite
E) sodium sulfide
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Combustion analysis of an unknown compound containing only carbon and hydrogen produced 2.277 g of CO2 and 1.161 g of H2O.What is the empirical formula of the compound?
A) CH2
B) C2H5
C) C4H10
D) C5H2
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the data given below to construct a Born-Haber cycle to determine the electron affinity of Br. DH°(kJ)
K(s) → K(g) 89
K(g) → K⁺(g) + e⁻ 419
Br2(l) → 2 Br(g) 193
K(s) + 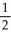 Br2(g) → KBr(s) -394
KBr(s) → K⁺(g) + Br⁻(g) 674
Br2(g) → KBr(s) -394
KBr(s) → K⁺(g) + Br⁻(g) 674
A) -885 kJ
B) -325 kJ
C) +367 kJ
D) -464 kJ
E) +246 kJ
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 202
Related Exams