A) 238 pm
B) 301 pm
C) 130 pm
D) 89.0 pm
E) 157 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances should have the highest melting point?
A) CO2
B) SrS
C) Kr
D) F2
E) MgO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cesium has a radius of 272 pm and crystallizes in a body-centered cubic structure.What is the edge length of the unit cell?
A) 314 pm
B) 385 pm
C) 544 pm
D) 628 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the coordination number for a body-centered cubic cell.
A) 0
B) 6
C) 8
D) 10
E) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid for argon.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an X-ray beam strikes the surface of a crystal with a lattice spacing of 145 nm,it produces a maximum reflection at an angle of 41.0°.What is the wavelength of the X-ray with a value of n=1?
A) 130°
B) 164°
C) 158°
D) 190°
E) 167°
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Nickel has a face-centered cubic structure and has a density of 8.90 g/cm3.What is its atomic radius?
A) 125 pm
B) 249 pm
C) 353 pm
D) 997 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is considered an ionic solid?
A) (NH4) 2CO3
B) CBr4
C) SeBr2
D) XeF4
E) None of these is an ionic solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium crystallizes in a body-centered cubic structure.What is the coordination number of each atom?
A) 4
B) 6
C) 8
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diamond crystallizes in a cubic lattice with an edge length of 357 pm.If there are a total of 8 carbon atoms in the unit cell,what is the density of diamond?
A) 12.9 g/cm3
B) 3.54 g/cm3
C) 8.26 g/cm3
D) 2.26 g/cm3
E) 6.20 g/cm3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the addition polymer formed from the compound below? CH2=CH-CH3
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 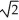 r.
r.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following forms an ionic solid?
A) Ag
B) C7H15NH2
C) Rb I
D) S O3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the edge length of a face-centered cubic unit cell made up of atoms having a radius of 128 pm?
A) 181 pm
B) 362 pm
C) 512 pm
D) 1020 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid for ice.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Identify the type of solid for AgCl.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is considered an atomic solid?
A) F2
B) CsBr
C) N2
D) Nb
E) None of these is an atomic solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vanadium crystallizes in a body centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 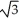 .
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an X-ray beam with a wavelength of 180 pm strikes the surface of a crystal,it produces a maximum reflection at an angle of 54.0°.If n=1,what is the separation between layers of atoms in the crystal?
A) 151 nm
B) 38.9 mm
C) 111 nm
D) 55.3 nm
E) 83.5 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of a polymer?
A) polyvinyl chloride
B) polystyrene
C) nylon
D) polyurethane
E) all of the above
Correct Answer

verified
E
Correct Answer
verified
Showing 1 - 20 of 43
Related Exams