A) 27.0
B) 9.00
C) 1.19 × 103
D) 2.90 × 105
E) 3.57 × 103
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following metals,if coated onto iron,would prevent the corrosion (oxidation) of iron?
A) Zn
B) Cu
C) Ag
D) Au
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Cu(s) ∣ Cu2+(aq,0.0032 M) ∣∣ Cu2+(aq,4.48 M) ∣ Cu(s)
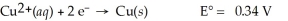
A) 0.00 V
B) 0.093 V
C) 0.34 V
D) 0.186 V
E) 0.052 V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidizing agent in the redox reaction represented by the following cell notation? Sn(s) ∣ Sn2+(aq) ∣∣ Ag+(aq) ∣ Ag(s)
A) Sn(s)
B) Ag+(aq)
C) Sn2+(aq)
D) Ag(s)
E) Pt
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the balanced equation for the galvanic cell reaction expressed using shorthand notation below? Ni(s) ∣ Ni2+(aq) ∣∣ Cl2(g) ∣ Cl-(aq) ∣ C(s)
A) Ni(s) + 2 Cl-(aq) → Ni2+(aq) + Cl2(g)
B) Ni(s) + Cl2(g) → Ni2+(aq) + 2 Cl-(aq)
C) Ni2+(aq) + 2 Cl-(aq) → Ni(s) + Cl2(g)
D) Ni2+(aq) + 2 Cl-(aq) → NiCl2(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
Mg(s) + Cu2+(aq) → Cu(s) + Mg2+(aq)

A) 2.04 V
B) -2.04 V
C) 2.72 V
D) -1.36 V
E) 1.36 V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that E°red = -1.66 V for Al3+/Al at 25°C,find E° and E for the concentration cell expressed using shorthand notation below. Al(s) ∣ Al3+(1.0 × 10-5 M) ∣∣ Al3+(0.100 M) ∣ Al(s)
A) E° = 0.00 V and E = +0.24 V
B) E° = 0.00 V and E = +0.12 V
C) E° = -1.66 V and E = -1.42 V
D) E° = -1.66 V and E = -1.54 V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of aluminum can be plated onto an object in 755 minutes at 5.80 A of current?
A) 73.5 g
B) 24.5 g
C) 220.g
D) 147 g
E) 8.17 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Sn(s) ∣ Sn2+(aq,0.022 M)  Ag+(aq,2.7 M) ∣ Ag(s)
Ag+(aq,2.7 M) ∣ Ag(s)
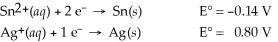
A) 1.01 V
B) -0.83 V
C) 1.31 V
D) 0.01 V
E) -0.66 V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -Q < K
A) Ecell = E°cell
B) Ecell = 0
C) E°cell < 0
D) Ecell < 0
E) E°cell > 0
F) Ecell > 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the criteria for a nonspontaneous reaction.
A) ΔG° < 0,E°cell > 0,K > 1
B) ΔG° > 0,E°cell > 0,K > 1
C) ΔG° > 0,E°cell < 0,K > 1
D) ΔG° < 0,E°cell > 0,K < 1
E) ΔG° > 0,E°cell < 0,K < 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
3 I2(s) + 2 Fe(s) → 2 Fe3+(aq) + 6 I⁻(aq)
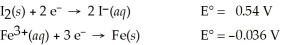
A) 3.5 × 10-59
B) 1.1 × 1017
C) 2.4 × 1058
D) 8.9 × 10-18
E) 1.7 × 1029
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the tabulated half-cell potentials below to calculate ΔG° for the following redox reaction.
2 Al(s) + 3 Mg2+(aq) → 2 Al3+(aq) + 3 Mg(s)

A) 4.1 × 102 kJ
B) 1.4 × 102 kJ
C) -2.3 × 102 kJ
D) -7.8 × 102 kJ
E) 6.8 × 102 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the type of battery that most cars contain.
A) lead-acid battery
B) dry-cell battery
C) alkaline battery
D) nickel-cadmium battery
E) nickel-metal hydride battery
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the following redox reaction if it occurs in acidic solution.What are the coefficients in front of H2C2O4 and H2O in the balanced reaction? MnO4⁻(aq) + H2C2O4(aq) → Mn2+(aq) + CO2(g)
A) H2C2O4 = 5,H2O = 8
B) H2C2O4 = 1,H2O = 1
C) H2C2O4 = 5,H2O = 1
D) H2C2O4 = 1,H2O = 4
E) H2C2O4 = 3,H2O = 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the following information, Cl2(g) + 2 e- → 2 Cl-(aq) E° = +1.36 V Mg2+(aq) + 2 e- → 2 Mg(s) E° = -2.37 V Which of the following chemical species is the strongest reducing agent?
A) Cl2(g)
B) Mg2+(aq)
C) Cl-(aq)
D) Mg(s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify oxidation.
A) increase in oxidation number
B) loss of electrons
C) gain of electrons
D) decrease in oxidation number
E) both A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -ΔG° < 0
A) Ecell = E°cell
B) Ecell = 0
C) E°cell < 0
D) Ecell < 0
E) E°cell > 0
F) Ecell > 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A voltaic cell is constructed with two Zn2+-Zn electrodes,where the half-reaction is Zn2+(aq) + 2e- → Zn(s) E° = -0.763 V The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10-2 M,respectively.The cell emf is ________ V.
A) -1.54 × 10-3
B) -378
C) 0.0798
D) 0.160
E) -0.761
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the reducing agent in the redox reaction represented by the following cell notation? Ni(s) ∣ Ni2+(aq) ∣∣ Ag+(aq) ∣ Ag(s)
A) Ni(s)
B) Ni2+(aq)
C) Ag+(aq)
D) Ag(s)
E) Pt
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 115
Related Exams