A) 248
B) -32
C) -13
D) -298
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solid form of matter in which there is long range repeating order is called ________.
A) amorphous
B) rigid
C) crystalline
D) fixed
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Chemical properties of a substance are those that can be observed without changing the composition of a substance.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is FALSE?
A) Matter may be a pure substance or it may be a mixture.
B) A pure substance may either be an element or a compound.
C) A mixture may be either homogeneous or heterogeneous.
D) Mixtures may be composed of two or more elements,two or more compounds,or a combination of both.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT an example of matter?
A) a pencil eraser
B) a balloon full of helium
C) a dust particle
D) heat from a burning candle
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A melting scoop of ice cream is an example of an exothermic process.
Correct Answer

verified
Correct Answer
verified
True/False
Matter is defined as anything that is visible to the human eye.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a solid piece of shiny sodium metal is exposed to chlorine gas,a large amount of heat is released and the white solid sodium chloride (table salt) forms.Based on this information,which of the following statements is TRUE?
A) This process represents a physical change.
B) Mass is lost during this process.
C) Sodium chloride is an element.
D) This process was exothermic.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The melting of ice is a physical change.
Correct Answer

verified
Correct Answer
verified
True/False
Temperatures reported in the Kelvin scale cannot be negative.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which state of matter has indefinite shape and is compressible?
A) liquid
B) solid
C) gas
D) plasma
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The amount of heat energy needed to increase the temperature of an object will vary depending on the heat capacity of the object.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the value of 23°C on the Fahrenheit scale?
A) 73
B) 52
C) 59
D) 81
Correct Answer

verified
Correct Answer
verified
True/False
Like mass,energy can neither be created nor destroyed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many calories are there in a 255 Calorie snack bar?
A) 2.55 × 105
B) 1.07 × 103
C) 60.9
D) 1 × 103
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order,what is the freezing point,room temperature and boiling point of water according to the Celsius scale?
A) 32-75-212
B) 0-75-100
C) 0-25-100
D) 0-298-373
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the following list of substances and heat capacities,choose the one that will have the lowest temperature after absorbing 100.0 kJ of heat.Assume identical masses of each substance start at the same initial temperature.
A) lead-0.128 J/g∙°C
B) copper-0.385 J/g∙°C
C) ethanol-2.42 J/g∙°C
D) water-4.18 J/g∙°C
E) not enough information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you hold a solid piece of pure gallium metal in your hand,your body heat will melt the gallium into its liquid form.This illustrates which of the following?
A) distillation
B) physical change
C) chemical change
D) chemical property
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Gases are the only form of matter that is easily compressible.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of water when supplied with 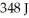 of heat will gain a temperature of 5.2°C?
of heat will gain a temperature of 5.2°C?
A) 15
B) 17
C) 19
D) 16
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 118
Related Exams