A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cyclohexane (C6H12) undergoes a molecular rearrangement in the presence of AlCl3 to form methylcyclopentane (MCP) according to the equation: C6H12 ⇌ MCP If Kc = 0.143 at 25 °C for this reaction,predict the direction in which the system will shift if the initial concentrations of C6H12 and MCP are 0.0400 mol L-1 and 0.0200 mol L-1,respectively.The system:
A) will shift left
B) will shift right
C) is already at equilibrium
D) is not at equilibrium and will remain in an unequilibrated state
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: N2(g) + 2 O2(g) ⇌ 2 NO2(g) ,Kc = 8.3 × 10-10 M-1 at 25 °C.What is the concentration of N2 gas at equilibrium when the concentration of NO2 is five times the concentration of O2 gas?
A) 3.3 × 10-11 mol L-1
B) 1.7 × 10-10 mol L-1
C) 6.0 × 109 mol L-1
D) 3.0 × 1010 mol L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
0.75 mol of N2 and 1.20 mol of H2 are placed in a 3.0 liter container.When the reaction N2(g) + 3 H2(g) ⇌ 2 NH3(g) reaches equilibrium,[H2] = 0.100 M.Which of the following is true?
A) [NH3] = 0.150 M
B) [NH3] = 0.200 M
C) [N2] = 0.650 M
D) [N2] = 0.250 M
E) [NH3] = [H2] = 0.05 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2 NO2(g) ⇌ N2O4(g) Kp equals ________.
A) Kc
B) RT/Kc
C) Kc(RT)
D) Kc/RT
E) Kc(RT) 2
Correct Answer

verified
Correct Answer
verified
True/False
The value of the equilibrium constant for a given reaction depends on the initial concentrations of reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following reactions, 2 PCl3(g) ⇌ 2 P(g) + 3 Cl2(g) Kc = 0.0667 PCl3(g) + Cl2(g) ⇌ PCl5(g) Kc = 4.0 Calculate Kc for the reaction below. 2 P(g) + 5 Cl2(g) ⇌ 2 PCl5(g)
A) 240
B) 1.1
C) 60
D) 23
E) 0.41
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorus trichloride and phosphorus pentachloride equilibrate in the presence of molecular chlorine according to the following reaction:
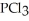 (g) +
(g) + 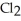 (g) →
(g) → 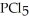 (g)
An equilibrium mixture at 450 K contains
(g)
An equilibrium mixture at 450 K contains 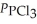 = 0.124 bar,
= 0.124 bar, 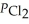 = 0.157 bar,and
= 0.157 bar,and 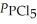 = 1.30 bar.
What is the value of Kp at this temperature?
= 1.30 bar.
What is the value of Kp at this temperature?
A) 66.8
B) 1.50 × ![]()
C) 2.53 × 10-2
D) 1.02
E) 4.63
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two moles of NH3 are initially present for the reaction: 2 NH3(g) ⇌ N2(g) + 3 H2(g) At equilibrium there is 1.00 mol NH3.How many moles of H2 are present at equilibrium?
A) 3.00 moles
B) 1.00 moles
C) 1.50 moles
D) 0.67 moles
E) 0.75 moles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Equilibrium constant K is constant except when one varies the:
A) concentrations of the reactants
B) temperature of the reaction
C) concentration of the products
D) partial pressures of the reactants
E) K always remains constant
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2 SO2 (g) + O2 (g) ⇌ 2 SO3 (g) ,Kc = 2.8 × 102 at 1000 K.If a vessel is filled with these gases such that the initial concentrations are [SO2] = 0.025 M,[O2] = 0.035 M,and [SO3] = 0.046 M,in which direction will a reaction occur and why?
A) toward products because Qc = 53
B) toward reactants because Qc = 0.019
C) toward products because Qc = 97
D) toward reactants because Qc = 2.8 × 103
E) it is at equilibrium because Qc = 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the equation:
![Phosphorus pentachloride decomposes to phosphorus trichloride at high temperatures according to the equation: At 250 °C 0.125 mol L<sup>-1</sup> PCl<sub>5</sub> is added to the flask.If K<sub>c</sub> = 1.80,what are the equilibrium concentrations of each gas? A) [PCl<sub>5</sub>] = 0.00765 mol L<sup>-1</sup>,[PCl<sub>3</sub>] = 0.117 mol L<sup>-1</sup>,and [Cl<sub>2</sub>] = 0.117 mol L<sup>-1</sup> B) [PCl<sub>5</sub>] = 0.0625 mol L<sup>-1</sup>,[PCl<sub>3</sub>] = 0.335 mol L<sup>-1</sup>,and [Cl<sub>2</sub>] = 0.335 mol L<sup>-1</sup> C) [PCl<sub>5</sub>] = 1.80 mol L<sup>-1</sup>,[PCl<sub>3</sub>] = 1.80 mol L<sup>-1</sup>,and [Cl<sub>2</sub>] = 1.80 mol L<sup>-1</sup> D) [PCl<sub>5</sub>] = 3.96 mol L<sup>-1</sup>,[PCl<sub>3</sub>] = 3.83 mol L<sup>-1</sup>,and [Cl<sub>2</sub>] = 3.83 mol L<sup>-1</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB5343/11ea7a5e_3e68_03ac_89bd_fbaa07e72cec_TB5343_11.jpg) At 250 °C 0.125 mol L-1 PCl5 is added to the flask.If Kc = 1.80,what are the equilibrium concentrations of each gas?
At 250 °C 0.125 mol L-1 PCl5 is added to the flask.If Kc = 1.80,what are the equilibrium concentrations of each gas?
A) [PCl5] = 0.00765 mol L-1,[PCl3] = 0.117 mol L-1,and [Cl2] = 0.117 mol L-1
B) [PCl5] = 0.0625 mol L-1,[PCl3] = 0.335 mol L-1,and [Cl2] = 0.335 mol L-1
C) [PCl5] = 1.80 mol L-1,[PCl3] = 1.80 mol L-1,and [Cl2] = 1.80 mol L-1
D) [PCl5] = 3.96 mol L-1,[PCl3] = 3.83 mol L-1,and [Cl2] = 3.83 mol L-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture containing 0.0392 M A(g) and 0.0452 M B(g) is allowed to come to equilibrium at 300 K.The reaction: 3 A(g) + 2 B(g) ⇌ C(g) + D(g) occurs.At equilibrium,[C] = 0.00128 M.What is the value of Kc?
A) 2.13 × 10-3
B) 0.849
C) 20.4
D) 4.91 × 10-2
E) 470
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: CH4(g) + 4 Cl2(g) ⇌ CCl4(l) + 4 HCl(g) ,ΔrH° = -398 kJ/mol The equilibrium is displaced to the right if:
A) the temperature is raised
B) the pressure is lowered
C) some carbon tetrachloride is removed
D) some hydrogen chloride is added
E) some chlorine gas is removed
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find Kp for the following reaction at 25.0 °C. SbCl5(g) ⇌ SbCl3(g) + Cl2(g) Kc = 2.51 × 10-2
A) 0.614
B) 1.03 × 10-3
C) 5.15 × 10-2
D) 9.74 × 102
E) 39.8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium constant expression for the following reaction: N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the isomerization reaction: Butane ⇌ isobutane Kp equals 25.0 at 500 °C.If the initial pressures of butane and isobutane are 20.0 atm and 0.0 atm,respectively,what are the pressures of the two gases at equilibrium?
A) P(butane) = 0.77 atm and P(isobutane) = 19.2 atm
B) P(butane) = 0.80 atm and P(isobutane) = 20.atm
C) P(butane) = 19.2 atm and P(isobutane) = 0.77 atm
D) P(butane) = 20 atm and P(isobutane) = 0.80 atm
Correct Answer

verified
Correct Answer
verified
True/False
The concentration of a pure solid is left out of a equilibrium constant expression but a pure liquid is included.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction: 3 Fe(s) + 4 H2O(g) ⇌ Fe3O4(s) + 4 H2(g) what is the effect of adding Fe(s) ?
A) The reaction shifts to the right.
B) There is no change.
C) The reaction shifts to the left.
D) Kp is decreased.
E) Kp is doubled.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction PCl5 (g) ⇌ PCl3 (g) + Cl2 (g) Kc = 0.0454 at 261 °C.If a vessel is filled with these gases such that the initial concentrations are [PCl5] = 0.20 M,[PCl3] = 0.20 M,and [Cl2] = 2.5 M,in which direction will a reaction occur and why?
A) toward products because Qc = 0.56
B) toward reactants because Qc = 2.5
C) toward products because Qc = 2.8
D) toward reactants because Qc = 0.0454
E) it is at equilibrium because Qc = 1
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 118
Related Exams