A) The isotope has 25 nucleons.
B) The isotope has 25 protons.
C) The isotope has 25 neutrons.
D) The isotope has 15 orbital electrons.
E) The isotope has 15 protons.
F) The isotope has 10 neutrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The primary source of the energy radiated by a star,such as the sun,is
A) beta decay.
B) alpha decay.
C) fission reactions involving uranium.
D) fusion reactions in which hydrogen is fused to form helium.
E) fusion reactions in which helium is fused to form iron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Going from medium mass nuclei to heavy nuclei,the average binding energy per nucleon
A) decreases.
B) behaves randomly with no clear pattern.
C) does not change.
D) increases.
E) doubles.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The material used in certain nuclear bombs is 239Pu,which has a half-life of about 20,000 years.How long must we wait for a buried stockpile of this substance to decay to 4.0% of its original 239Pu mass?
A) 93,000 y
B) 64,000 y
C) 45,000 y
D) 800 y
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The reaction 2H + 2H →? 3H + 1H releases 4.03 MeV of energy.If 1.0 kg of deuterium were to go through this reaction,how much energy would be produced? 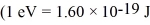 ,
, 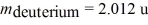 ,1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg)
,1 u = 931.5 MeV/c2 = 1.6605 × 10-27 kg)
A) 9.7 × 107 J
B) 9.7 × 1013 J
C) 1.9 × 107 J
D) 1.9 × 1014 J
E) 1.9 × 1011 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of energy that is released in the fusion reaction 2H + 2H → 4He,given the masses: 2H: 2.014102 u 4He: 4.002603 u (1 u = 931.5 MeV/c2)
A) 24 MeV
B) 18 MeV
C) 13 MeV
D) 12 MeV
E) 36 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a 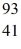 Nb atom,the number of protons,neutrons,and electrons in the atom is
Nb atom,the number of protons,neutrons,and electrons in the atom is
A) 41, 52, 93.
B) 41, 52, 52.
C) 41, 52, 41.
D) 41, 52, 0.
E) 52, 41, 0.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider two different isotopes of the same neutral element.Which statements about these isotopes are true? (There may be more than one correct choice.)
A) Both isotopes contain the same number of neutrons.
B) Both isotopes contain the same number of protons.
C) Both isotopes contain the same number of nucleons.
D) Both isotopes contain the same number of orbital electrons.
E) The sum of the protons and neutrons is the same for both isotopes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A radioactive nuclide of atomic number Z emits an electron,then the daughter nuclide emits a gamma ray.What is the atomic number of the resulting nuclide after both processes?
A) Z + 1
B) Z - 1
C) Z - 2
D) Z - 3
E) Z + 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass of 14C (having a half-life of 5730 years) do you need to provide a decay rate of 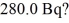 (1 u = 1.6605 × 10-27 kg)
(1 u = 1.6605 × 10-27 kg)
A) 1.70 × 10-12 kg
B) 5.38 × 10-19 kg
C) 3.84 × 10-20 kg
D) 8.68 × 10-13 kg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An excited 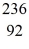 U* nucleus undergoes fission into two fragments,as shown:
U* nucleus undergoes fission into two fragments,as shown: 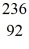 U* →
U* → 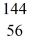 Ba +
Ba + 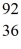 Kr The following atomic masses are known:
Kr The following atomic masses are known: 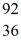 Kr: 91.926270 u
Kr: 91.926270 u 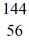 Ba: 143.922845 u
Ba: 143.922845 u 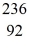 U*: 236.045563 u
Assume,at a given instant,that the two fission fragments are spherical,just barely in contact,and carry spherically symmetric charge distributions.At that instant,what is the electrostatic interaction energy of the two fragments,in MeV? (1 u = 1.6605 × 10-27 kg = 9
U*: 236.045563 u
Assume,at a given instant,that the two fission fragments are spherical,just barely in contact,and carry spherically symmetric charge distributions.At that instant,what is the electrostatic interaction energy of the two fragments,in MeV? (1 u = 1.6605 × 10-27 kg = 9 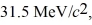
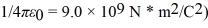
A) 230 MeV
B) 240 MeV
C) 250 MeV
D) 260 MeV
E) 270 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The stability of 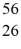 Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known:
Fe with respect to alpha,β+,and β- decay is to be determined.Do not consider the possibility of decay by electron capture.The following atomic masses are known: 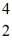 He: 4.002603 u
He: 4.002603 u 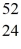 Cr: 51.944768 u
Cr: 51.944768 u 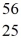 Mn: 55.938907 u
Mn: 55.938907 u 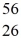 Fe: 55.934939 u
Fe: 55.934939 u 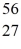 Co: 55.939841 u The
Co: 55.939841 u The 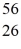 Fe nuclide is
Fe nuclide is
A) not subject to alpha, β+, or β- decay.
B) subject to alpha decay only.
C) subject to β+decay only.
D) subject to β- decay only.
E) subject to β+ or β- decay, but not to alpha decay.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The maximum permissible workday dose for occupational exposure to radiation is 26 mrem.A 55-kg laboratory technician absorbs 3.3 mJ of 0.40-MeV gamma rays in a workday.The relative biological efficiency (RBE) for gamma rays is 1.00.What is the ratio of the equivalent dosage received by the technician to the maximum permissible equivalent dosage?
A) 0.23
B) 0.25
C) 0.28
D) 0.30
E) 0.32
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioactive nuclei 60Co is widely used in medical applications.It undergoes beta decay,and the total energy of the decay process is 2.82 MeV per decay event.The half-life of this nucleus is 272 days.Suppose that a patient is given a dose of 6.9 µCi of 60Co.If all of this material decayed while in the patient's body,what would be the total energy deposited there? 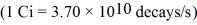
A) 11 J
B) 8.6 GJ
C) 3.9 J
D) 24 J
E) 4.15 MJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In massive stars,three helium nuclei fuse together,forming a carbon nucleus.This reaction heats the core of the star.The net mass of the three helium nuclei must therefore be
A) higher than that of the carbon nucleus.
B) less than that of the carbon nucleus.
C) the same as that of the carbon nucleus since mass is always conserved.
D) the same as that of the carbon nucleus since energy is always conserved.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Today,the uranium found on Earth contains 0.720% 235U (with a half-life of 0.700 billion years) and 99.28% 238U (with a half-life of 4.50 billion years) .At a time 2.20 billion years ago,what percent of the uranium on Earth was 238U (assuming that no other uranium isotopes were present) ?
A) 95.6%
B) 2.18%
C) 6.29%
D) 8.68%
E) 4.53%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutral deuterium atom,  H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the
H,has a mass of 2.014102 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.What is the binding energy of the  H nucleus?
H nucleus? 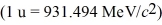
A) 1.1 MeV
B) 1.7 MeV
C) 2.2 MeV
D) 2.9 MeV
E) 3.4 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the binding energy per nucleon for 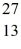 Al? The neutral
Al? The neutral 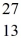 Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
Al atom has a mass of 26.981539 u; a neutral hydrogen atom has a mass of 1.007825 u; a neutron has a mass of 1.008665 u; and a proton has a mass of 1.007277 u.(1 u = 931.494 MeV/c2)
A) 8.3 MeV
B) 6.7 MeV
C) 5.4 MeV
D) 3.4 MeV
E) 2.8 MeV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Plutonium-239 decays into uranium-235 plus an alpha particle.The energy released in the process is 5.24 MeV.Given the following mass values 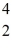 He: 4.002603 u
He: 4.002603 u 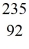 U: 235.043924 u what is the mass of
U: 235.043924 u what is the mass of 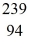 Pu in atomic mass units? (1 u = 931.494 MeV/c2)
Pu in atomic mass units? (1 u = 931.494 MeV/c2)
A) 239.05215 u
B) 239.02775 u
C) 239.00189 u
D) 238.99919 u
E) 238.98884 u
Correct Answer

verified
Correct Answer
verified
True/False
The iron nucleus has the greatest binding energy of any nucleus.
Correct Answer

verified
False
Correct Answer
verified
Showing 1 - 20 of 90
Related Exams