Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxygen in the air we breath is classified as:
A) the solute in a homogeneous gas mixture.
B) the solvent in a homogeneous gas mixture.
C) the solute in a heterogeneous gas-liquid mixture.
D) the solvent in a simple mixture.
E) none of the above
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
How many milliliters of 0.755 M H2SO4 solution is needed to react with 55.0 mL of 2.50 M KOH solution? Given: 2 KOH (aq) + H2SO4 (aq) → 2 H2O (l) + K2SO4 (aq)
A) 51.9 mL
B) 182 mL
C) 91.1 mL
D) 17200 mL
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A supersaturated solution is unstable and crystallization usually occurs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of Pb(NO3) 2 is 55 grams per 100 g H2O at 20°C.Which term would properly describe a solution where 44 grams of Pb(NO3) 2 is added to 100 grams of water at this temperature?
A) insoluble
B) unsaturated
C) saturated
D) supersaturated
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The fact that the oceans contain salt water shows that polar solvents dissolve ionic solutes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following aqueous solutions would be expected to freeze at the lowest temperature?
A) 1 molal KNO3
B) 1 molal NaCl
C) 1 molal CaCl2
D) 1 molal C6H12O6 (fructose)
E) All of these solutions would freeze at the same temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In comparing a 0.25 molality aqueous NaCl solution to a 0.25 molality aqueous CaCl2 solution:
A) the NaCl solution has the higher boiling point and the lower freezing point.
B) the CaCl2 solution has the higher boiling point and the lower freezing point.
C) the NaCl solution has the higher boiling point and the CaCl2 solution has the lower freezing point.
D) the CaCl2 solution has the higher boiling point and the NaCl solution has the lower freezing point.
E) both solutions have the same boiling point and the same freezing point.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
A solution contains 100.0 g water,10.0 g NaCl,and 15.0 g methanol.What is the mass percent of methanol in the solution?
A) 8.00%
B) 10.0%
C) 12.0%
D) 15.0%
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of solids in water:
A) is independent of pressure above solution.
B) increases with increasing pressure above solution.
C) decreases with increasing pressure above solution.
D) Solids are not soluble in water.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you prepare a solution by adding sufficient amount of solute so that after heating and cooling the solution there is a visible amount of solid solute left in the bottom of the beaker,the solution would be considered ________.
A) unsaturated
B) saturated
C) supersaturated
D) thermally saturated
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The solubility of gases in water decreases with increasing temperature.
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
Calculate the molarity of a KCl solution made by dissolving 24.7 g of KCl in a total volume of 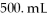 .
.
A) 0.331
B) 0.663
C) 0.166
D) 6.04
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final concentration of a solution prepared by diluting 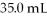 Of
Of 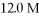 HCl to a final volume of
HCl to a final volume of 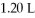 ?
?
A) 0.504 M
B) 3.50 M
C) 0.420 M
D) 0.350 M
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Vitamin C is a water-soluble vitamin,so it is likely that this vitamin is polar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume (L) of 2.00 M KCl solution contains 25.0 g of KCl?
A) 0.335
B) 0.168
C) 0.672
D) 1.49
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the change in the freezing point of a solution made by dissolving 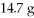 Of C6H12O6 into
Of C6H12O6 into 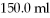 Of water? The density of water is
Of water? The density of water is 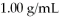 And
And 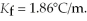
A) 0.152°C
B) 1.01°C
C) 18.97°C
D) 1.82°C
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of KCl are needed to make 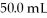 Of
Of 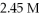 KCl?
KCl?
A) 9.13
B) 1.52
C) 91.3
D) 0.123
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a KCl solution prepared by dissolving 0.525 moles of KCl in 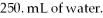
A) 0.476
B) 0.00210
C) 2.10
D) 2.02
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A sugar solution is an example of a weak electrolyte solution.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 122
Related Exams