A) 12.1 min
B) 6.03 min
C) 0.603 min
D) 12.7 min
E) 1.27 min
Correct Answer

verified
Correct Answer
verified
True/False
Fission is the process of combining small nuclei into larger nuclei.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have the greatest penetrating power through matter?
A) gamma rays
B) alpha particles
C) protons
D) beta particles
E) positron particles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the decay constant for 131I is 9.98 x 10-7 s-1, calculate the activity of a 1.00 mg sample.
A) 4.59 x 1012 atoms/s
B) 4.73 x 1012 atoms/s
C) 4.61 x 1024 atoms/s
D) 9.98 x 10-4 atoms/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) An isotope that will fission when hit by a neutron is said to be fertile.
B) A moderator slows down neutrons.
C) Control rods absorb energy to control the fission reaction.
D) Electrons dislodged from an atom or molecule by alpha or beta particles are called primary electrons.
E) Radiation exposes photographic film the same as light.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) Geiger-Müller counters detect radiation by the ionization caused in the gas in the counter.
B) Bubble chambers detect radiation by the ionization trail left by the radiation.
C) Radiation causes no harm to the human body.
D) Radiation kills cancer cells more easily than normal cells.
E) Alpha particles are less penetrating than beta particles.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
210Pb → 4He + ________
A) 214Pb
B) 206Hg
C) 214Hg
D) 206Pb
E) 206Br
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are true for alpha particles? I) α particles are equivalent to 2 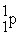 + 2
+ 2 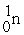 .
II) α decay decreases the atomic number by four.
III) α particles are deflected more strongly than e- by magnetic fields, but in the opposite direction.
IV) α particles are easily stopped as they strike matter.
.
II) α decay decreases the atomic number by four.
III) α particles are deflected more strongly than e- by magnetic fields, but in the opposite direction.
IV) α particles are easily stopped as they strike matter.
A) I and II
B) I, II, III
C) I and III
D) I and IV
E) I, II, III, and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive decay is:
A) temperature independent
B) a second-order process
C) a zero-order process
D) a process with a large activation energy
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of 238U, which decomposes to 206Pb, is 4.51 × 109 y. A rock contains equal masses of these two isotopes. How old is this rock?
A) 4.07 × 109 y
B) 4.06 × 109 y
C) 9.02 × 109 y
D) 4.51 × 109 y
E) 5.00 × 109 y
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The charcoal from ashes found in a cave gave 7.4 14C counts per gram per minute. Wood from the outer portion of a growing tree gives a comparable count of 15.3. The half-life of 14C is 5700 years. How old are the ashes?
A) 5700 y
B) 5437 y
C) 3245 y
D) 9220 y
E) 5970 y
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning alpha decay is INCORRECT?
A) It involves nuclides with atomic number larger than 83 and mass number larger than 200.
B) It has great penetrating power, but little ionizing power.
C) It often leaves the nucleus in an excited state.
D) The atomic number decreases by two.
E) The alpha particle is made up of two protons and two neutrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement.
A) Radioactive 32P as a phosphate can be used to trace the uptake of phosphorus in plants.
B) The thyroid gland can be studied by the use of radioactive iodine.
C) There is no difference in physical properties between isotopes.
D) The mechanism of a chemical reaction can be followed by a radioactive tracer such as 35S.
E) Industrial catalysts can be followed by a tracer such as 192Ir.
Correct Answer

verified
Correct Answer
verified
True/False
Radioactive decay series decay by alpha and positron emission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct word to describe the nuclear stability of the following isotope. If it is radioactive, choose the most likely mode of decay. 238U
A) radioactive, beta
B) radioactive, fission
C) radioactive, alpha
D) radioactive, neutron
E) stable
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct word to describe the nuclear stability of the following isotope. If it is radioactive, choose the most likely mode of decay. 197Au
A) radioactive, beta
B) radioactive, fission
C) radioactive, alpha
D) radioactive, neutron
E) stable
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the case of radioactive element X, which decays by electron emission with a half-life of 4 days to a stable nuclide of element Z:
A) 2 g of element X is required to produce 1.5 g of element Z after 8 days
B) element Z will weigh considerably less than element X after decay is complete because of the loss of electrons
C) element Z will weigh more than element X when decay is complete since Z has the higher atomic number
D) after 8 days the sample will consist of one-fourth element Z and three-fourths element X
E) after 8 days element X will be all element Z
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the INCORRECT nuclear reaction.
A) ![]() +
+![]() →
→![]() +
+![]() + 3
+ 3![]()
B) ![]() +
+![]() →
→![]() +
+![]()
C) ![]() +
+![]() →
→![]() +
+![]()
D) ![]() +
+![]() →
→![]() +
+![]() +
+![]()
E) ![]() +
+![]() →
→![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct statement.
A) Instability leading to decay by alpha particle emission is found mostly in nuclides of very large mass number.
B) Decay by beta emission is confined to nuclides of very low mass number.
C) Ejection of an electron by a nuclide results in a nuclide of next lower mass number.
D) All radiations emitted by radioactive nuclides consist of charged, high energy particles.
E) Many alpha and beta emissions are followed by gamma emissions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The initial decay rate of a nuclide is 1.77 × 1012/m. After 7.63 d, the decay rate is 1.82 × 1011/m. What is the half-life of the isotope?
A) 139 d
B) 0.298 d
C) 0.207 d
D) 2.32 d
E) 2.50 d
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 100
Related Exams