A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which structure BEST describes the polarity of an H-O bond? 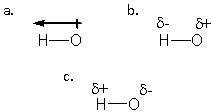
A) a
B) b
C) c
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A phosphorus atom with four bonds has a charge of:
A) +1.
B) +2.
C) -1.
D) -2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule will have the STRONGEST attraction to another molecule of its own kind? 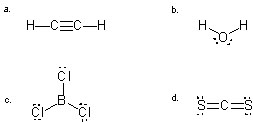
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Lewis structure,although acceptable,LEAST describes the carbonate ion? 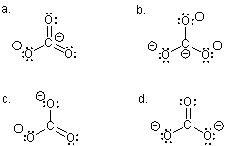
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electronic geometry around the central atom in CO2? 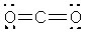
A) trigonal planar
B) linear
C) tetrahedral
D) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ozone has the formula O3.Which statement is CORRECT concerning ozone?
A) One oxygen atom has a +1 formal charge while another has a -1 formal charge.The third one is neutral.
B) All three atoms are neutral.
C) Two atoms have a -1 formal charge,and the third oxygen atom has a +1 formal charge.
D) Two atoms have a +1 formal charge,and the third oxygen atom has a -1 formal charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule(s) is/are likely to be a gas at room temperature due to a lack of attraction to another molecule of its own kind? 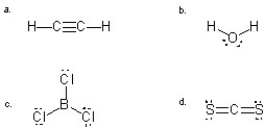
A) a,b,and c
B) a,c,and d
C) b
D) c and d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In this Lewis structure,which atom does NOT have a complete valence? 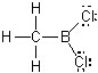
A) H
B) B
C) C
D) Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on the oxygen atom in this structure? 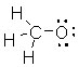
A) +1
B) +2
C) -1
D) -2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 30 of 30
Related Exams